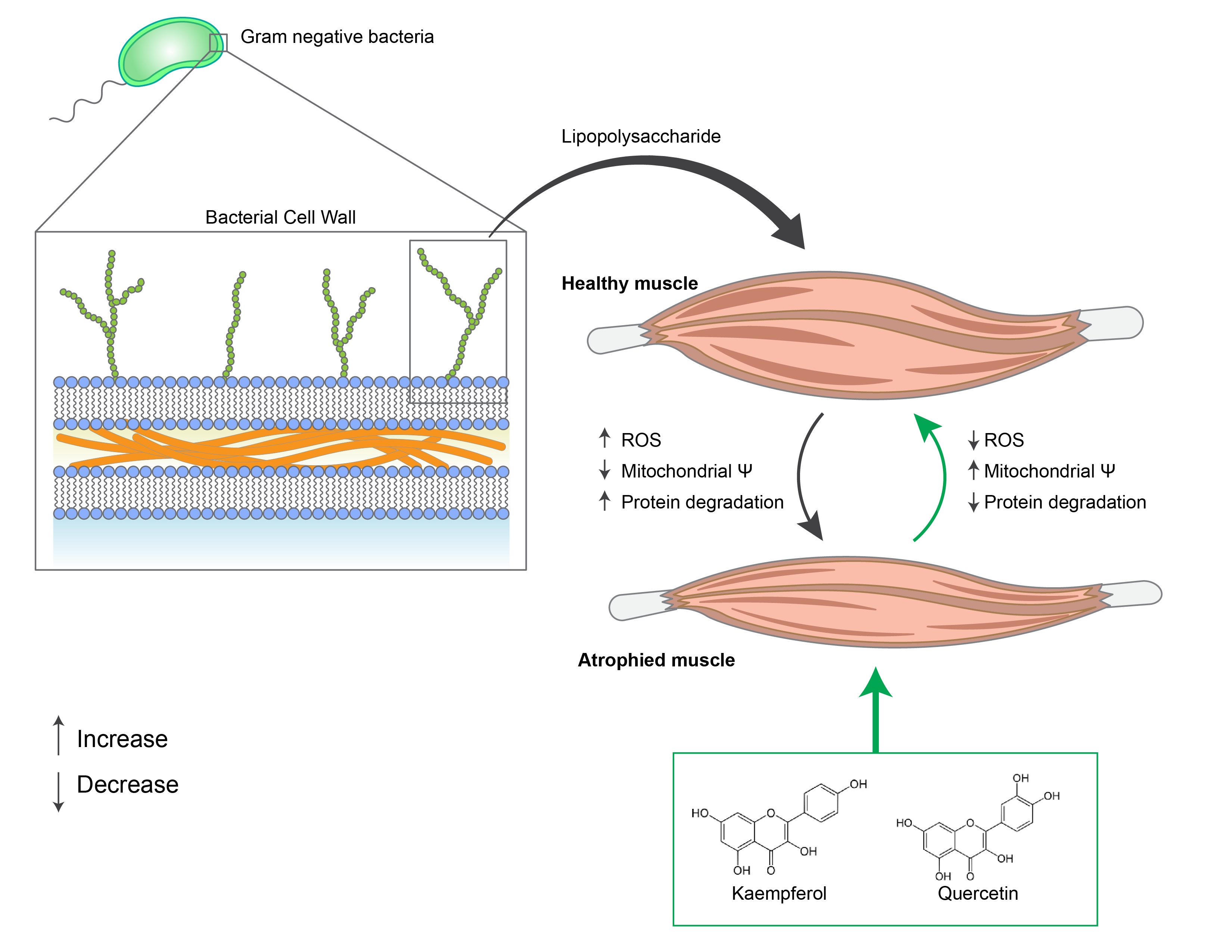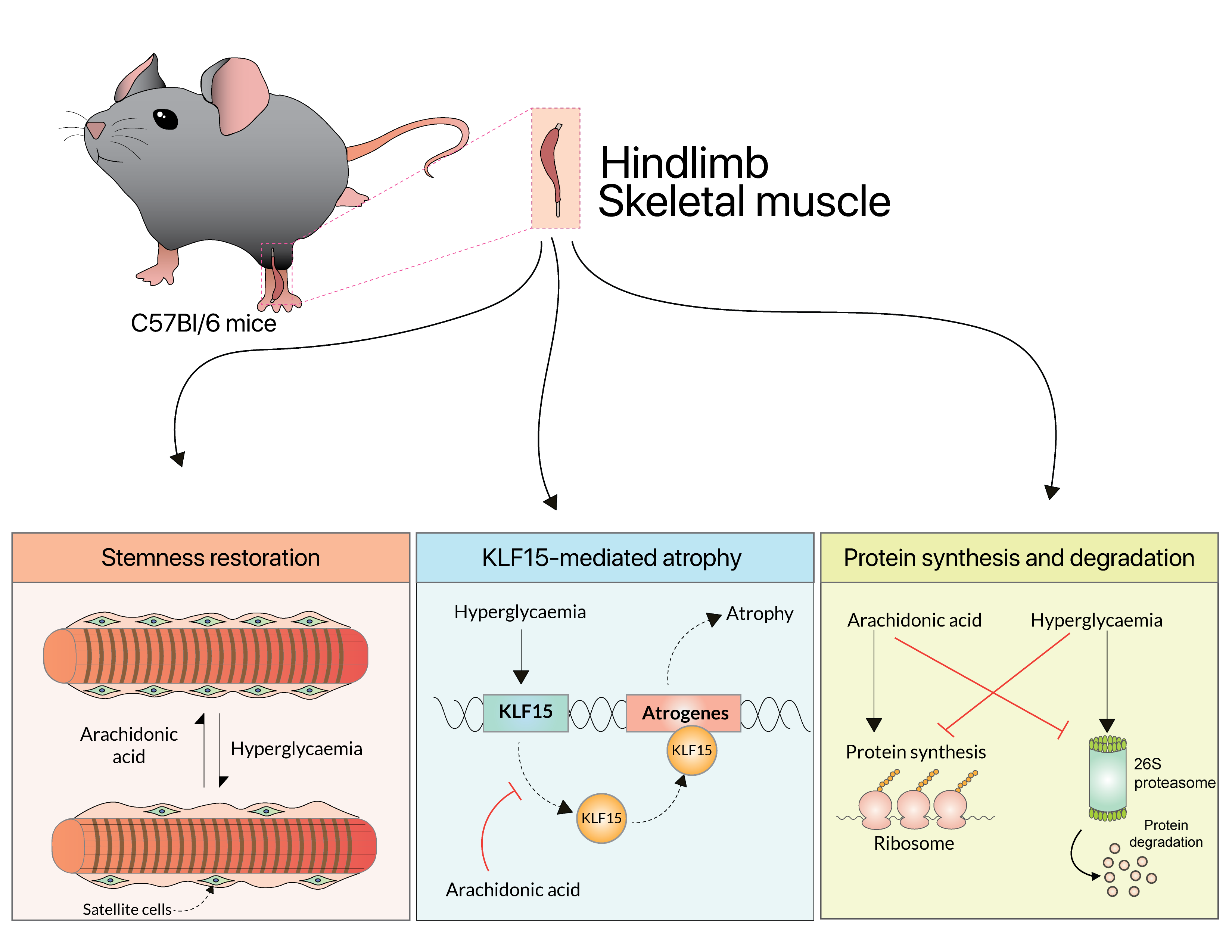
Arachidonic Acid Protects Skeletal Muscle Against Hyperglycaemia-Induced Muscle Atrophy by Modulating Myogenesis and Regulating KLF15 Expression in C57Bl/6 Mice
Published in The FASEB Journal (2025), this study investigates the protective role of arachidonic acid (AA) in mitigating hyperglycaemia-induced skeletal muscle atrophy using a streptozotocin-induced Type 1 diabetes mouse model. The findings demonstrate that short-term AA supplementation restores muscle mass and integrity by activating muscle stem cells (satellite cells) and reinstating the expression of myogenic regulators such as Pax7, MyoD, and Integrin-α7. Mechanistically, AA downregulates the atrophy-associated transcription factor KLF15, restores the NFκβ/AMPK signaling balance, and suppresses inflammation and proteolysis. Quantitative proteomic analysis further reveals that AA reverses diabetes-induced dysregulation of proteasomal, metabolic, and structural proteins, highlighting its ability to re-establish cellular homeostasis and promote myogenesis. Together, these results position arachidonic acid as a potent bioactive lipid capable of preserving skeletal muscle integrity and regenerative capacity under diabetic stress, offering new therapeutic insights into metabolic muscle disorders.
The FASEB Journal
Read more